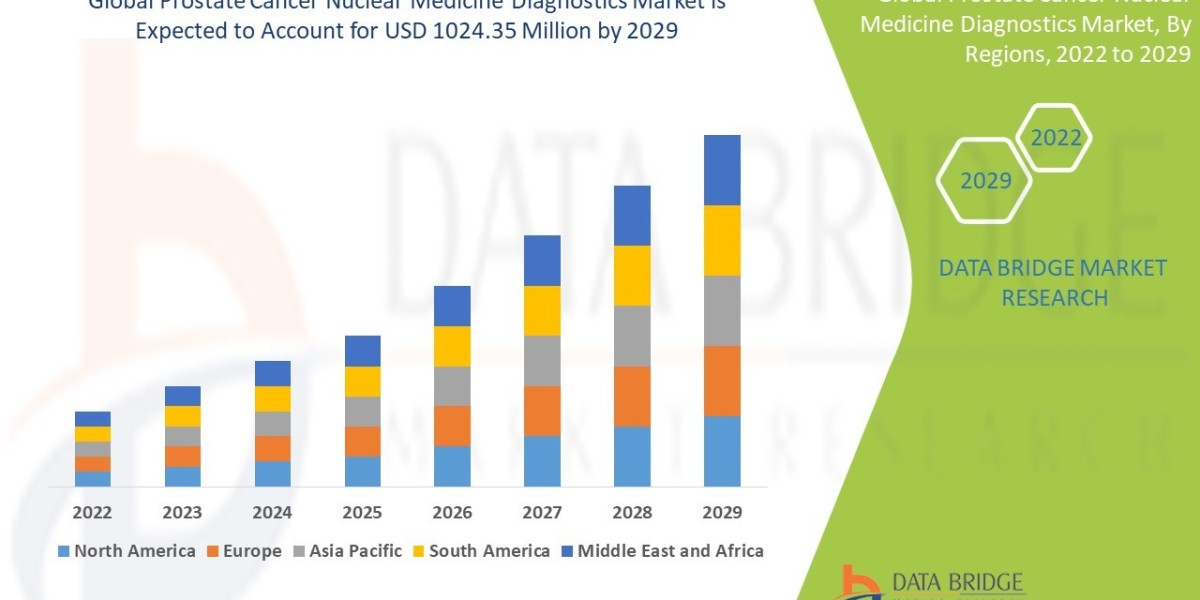
A significant rise in prostate cancer incidence and the accelerating adoption of highly specific diagnostic tools are propelling the global Prostate Cancer Nuclear Medicine Diagnostics Market toward a billion-dollar valuation. According to a recent analysis by Data Bridge Market Research, the market, valued at USD 389.14 million in 2021, is forecast to achieve a massive valuation of USD 1024.35 million by 2029. This robust expansion represents a compelling Compound Annual Growth Rate (CAGR of 11.7%) during the forecast period of 2022 to 2029.
The strong growth trajectory is fundamentally driven by the shift towards precision oncology, where early, accurate, and non-invasive detection is paramount to effective treatment planning and improved patient outcomes.
Get a Sample Report of Prostate Cancer Nuclear Medicine Diagnostics Market Forecast @https://www.databridgemarketresearch.com/request-a-sample?dbmr=global-prostate-cancer-nuclear-medicine-diagnostics-market
Market Dynamics, Segmentation, and Growth Drivers Prostate Cancer Nuclear Medicine Diagnostics
The market's acceleration is directly linked to technological advancements in radiopharmaceuticals and imaging equipment, which allow for the detection of prostate cancer recurrence and metastasis at earlier stages than conventional imaging techniques.
A primary driver of this market is the maturation of the theranostics approach—the combination of therapy and diagnostics—where a diagnostic agent is paired with a therapeutic counterpart. The associated key LSI growth area is the rapid integration of Prostate-Specific Membrane Antigen (PSMA) Imaging. PSMA-targeted radiopharmaceuticals are revolutionizing diagnostics by providing unparalleled specificity and sensitivity, enabling clinicians to visualize even small, previously undetectable lesions.
Segmentation across the market is driven primarily by:
Product Type: Including Technetium-99m, Fluorine-18, and Gallium-68-based radiotracers. The growing demand for advanced PET tracers (like F-18 and Ga-68) is contributing heavily to the market's value growth.
End-Users: Hospitals, diagnostic imaging centers, and specialized cancer research centers are the largest consumers, with hospitals retaining the dominant share due to high patient volumes and advanced infrastructure requirements.
Application: Driven by both initial diagnosis/staging and the critical area of monitoring disease recurrence, particularly in patients with rising PSA levels after initial treatment.
The increasing geriatric population and supportive government initiatives to improve cancer screening programs across developed and developing nations further bolster this strong CAGR forecast.
Do you have any specific queries or need any Prostate Cancer Nuclear Medicine Diagnostics Market Submit your inquiry here @https://www.databridgemarketresearch.com/inquire-before-buying?dbmr=global-prostate-cancer-nuclear-medicine-diagnostics-market
Competitive Landscape and Keyplayers Prostate Cancer Nuclear Medicine Diagnostics Market
The highly competitive Prostate Cancer Nuclear Medicine Diagnostics Market features several prominent global and regional players focused on R&D for next-generation radiopharmaceuticals and optimizing the supply chain for radioisotopes.
The major companies influencing the market's direction include:
Blue Earth Diagnostics (U.K.), Lantheus (U.S.), Theragnostics Ltd (U.K.), Jubilant Pharma Limited (U.S.), NCM-USA LLC (U.S.), Telix Pharmaceuticals Ltd (Australia), Cardinal Health (U.S.), General Electric (U.S.), Bayer AG (Germany), Bracco Diagnostic Inc. (Italy), NorthStar Medical Radioisotopes, LLC (U.S.), Eckert & Ziegler (Germany), Jubilant DraxImage, Inc. (Canada), PharmaLogic Holdings Corp. (U.S.), Institute of Isotopes Co., Ltd (Hungary), SHINE Medical Technologies, LLC (U.S.), and Global Medical Solutions LLC (China).
These companies are heavily invested in securing regulatory approvals for new tracers and forming strategic partnerships to ensure widespread distribution and accessibility of these time-sensitive diagnostics.
Get A Buy Now Report Prostate Cancer Nuclear Medicine Diagnostics Market Forecast @https://www.databridgemarketresearch.com/checkout/buy/global-prostate-cancer-nuclear-medicine-diagnostics-market/compare-licence
Future Outlook
The evidence overwhelmingly points to a robust and dynamic future for the Prostate Cancer Nuclear Medicine Diagnostics Market. With a compelling CAGR of 11.7% and a target valuation exceeding $1 billion by 2029, the sector is positioned to be a cornerstone of modern cancer care. The continued success of PSMA-based agents and the industry's commitment to advancing theranostics will not only drive financial growth but, more importantly, lead to better, more personalized treatment options for millions of patients globally. This market represents one of the most exciting and impactful areas within the medical diagnostics sector today.